
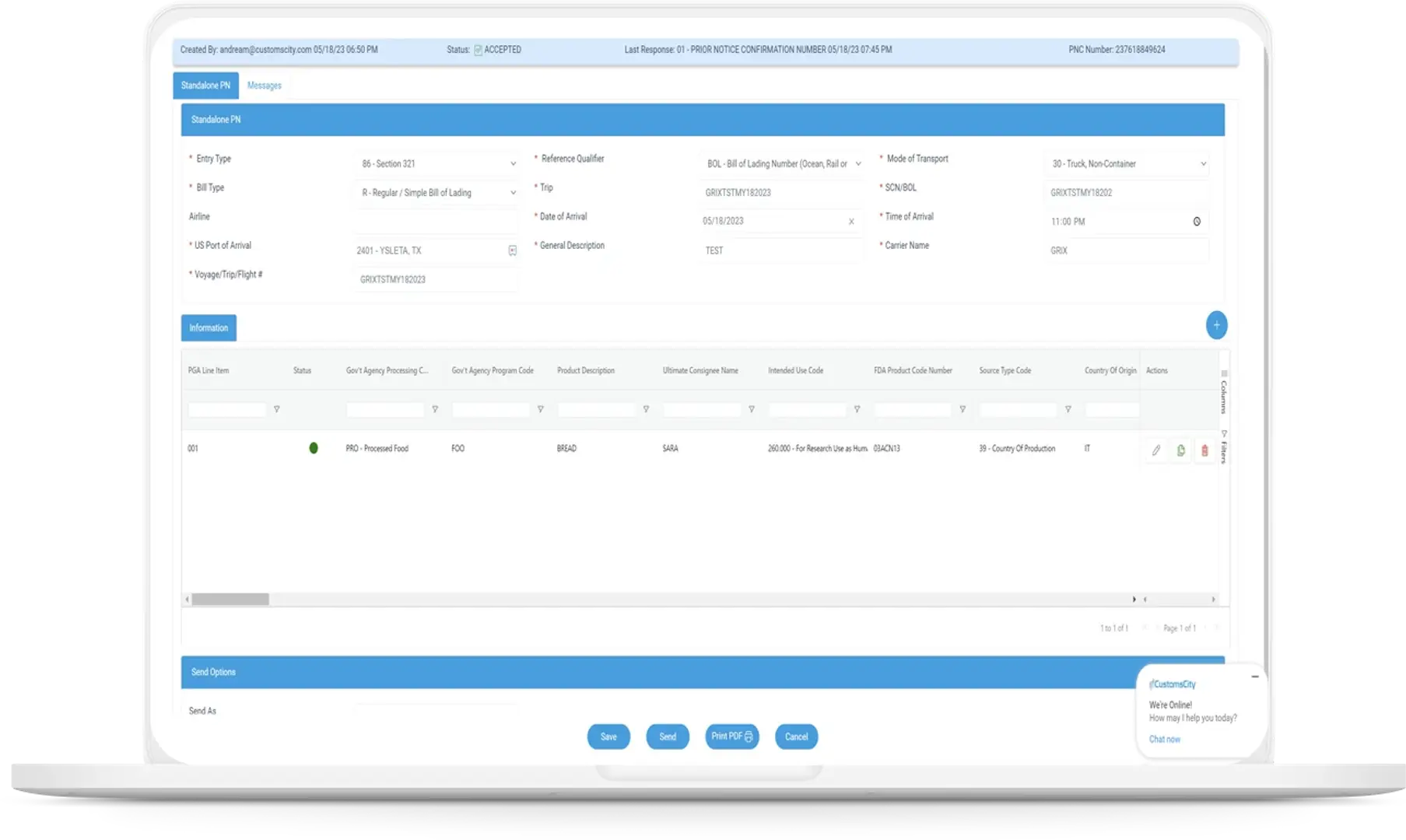
FDA Prior Notice Web Entry, US FDA Prior Notice Explained for Importers
The FDA Prior Notice Web Entry system is a mandatory process that importers must use to inform the U.S. Food and Drug Administration about incoming food shipments. It requires submitters to provide detailed information about the food products before they arrive at a U.S. port to ensure safety and regulatory compliance. This system helps the FDA assess any potential risks and prevent unsafe or adulterated food from entering the country. Importers or their agents must electronically submit and confirm prior notice within specified timeframes depending on the transportation mode. Failure to file accurate and timely prior notice can lead to shipment delays, inspections, or refusals, which increases costs and complicates supply chains. Understanding how to navigate the FDA Prior Notice Web Entry system is essential for smooth import operations.
The process requires careful attention to detail and adherence to FDA regulations outlined in Title 21 of the Code of Federal Regulations. For anyone involved in importing food or animal feed into the U.S., mastering these requirements offers clearer compliance and reduces the risk of enforcement actions.
Understanding FDA Prior Notice Web Entry
FDA Prior Notice Web Entry involves the electronic submission of detailed shipment information before the arrival of regulated products into the United States. This ensures the FDA screens imports efficiently to safeguard public health.The process requires specific data on the shipment, submitter, and product to be submitted within defined time frames. Compliance with these requirements is crucial to avoid shipment delays or refusals at the U.S. port of entry.
Definition and Purpose of FDA Prior Notice
FDA Prior Notice is an electronic notification system used to inform the U.S. Food and Drug Administration about shipments of regulated products before they reach U.S. borders. The purpose is to allow the FDA to evaluate potential risks and conduct timely inspections on imported goods. By receiving advance information, the FDA can prevent unsafe or adulterated products from entering the U.S. market. This process supports food safety and helps enforce regulations related to human and animal foods, dietary supplements, and other FDA-regulated products. The system improves transparency and coordination between importers, customs, and regulatory authorities, enhancing overall import control.
Legal Requirements for US FDA Prior Notice
Under Title 21 CFR Part 1, Subpart I, Prior Notice must be filed electronically for all relevant food and certain other products before shipment arrives in the United States. Failure to submit timely and accurate prior notice can result in shipment refusal or delays.The notice must be submitted before the earliest arrival time at the first U.S. port of entry. Deadlines vary depending on the mode of transportation—much earlier for air and sea shipments compared to land. The FDA must receive and confirm the submission electronically.
Proper submission requires detailed information about the shipment, including product description, quantity, manufacturer data, and shipment route. Meeting these legal requirements is mandatory for lawful importation.
Who Needs to Submit a Prior Notice
The responsibility to submit the Prior Notice lies with the importer or their agent, such as a customs broker or freight forwarder. The submitter must be knowledgeable about the shipment details and legally authorized to file on behalf of the importing party.This requirement applies to any entity bringing food, dietary supplements, animal food, or certain cosmetics regulated by the FDA into the U.S. If multiple parties are involved, the importer is ultimately accountable for compliance. Third-party logistics providers or consignee agents often assist but do not remove the importer’s duty to ensure the notice is filed correctly and in time.
Common Types of Shipments Requiring Web Entry
The FDA mandates Prior Notice for shipments containing:
• Food products for human consumption
• Animal food and feed ingredients
• Dietary supplements
• Certain cosmetic products regulated by the FDA
Shipments excluded typically involve products not regulated by the FDA or imports expressly exempt under existing regulations. Each shipment must be reported via the FDA Prior Notice Web Entry system, where importers provide specific details such as product description, manufacturer information, shipment origin, and expected arrival. Proper classification of shipments ensures smooth processing and compliance with FDA import rules.
How to Complete US FDA Prior Notice Submission
Submission for US FDA Prior Notice requires accurate data entry, meeting submission deadlines, and understanding the proper use of the Web Entry system. Proper documentation and timely filing ensure compliance and avoid shipment delays or refusals.
Step-by-Step Guide to Web Entry Submission
The user begins by accessing the FDA Prior Notice Web Entry system. They first enter shipment details such as shipper, consignee, and shipment identifier. Next, they provide specific product information, including food descriptions, quantity, and packaging. The system allows saving entries as drafts for review before final submission. Completing the Web Entry is essential; only then does the system send the notice to the FDA. After submission, no edits are possible, and draft entries are canceled automatically upon completion.
Required Data and Documentation
Required data includes the shipper’s and consignee’s details, shipment arrival port, and anticipated arrival date. Additionally, product specifics like the manufacturer, country of origin, and description of food items must be accurate. Documentation supporting compliance includes commercial invoices and bills of lading but is not uploaded in the web system. The submitter must ensure consistency between prior notice data and physical shipment. The accuracy and completeness of this data help the FDA assess food safety risk before the shipment’s arrival.
Timeline and Deadlines for Prior Notice
The FDA requires prior notice to be submitted electronically before food arrives at the first U.S. port. Timelines depend on the mode of transportation:
• Air and Rail: At least 4 hours before arrival.
• Truck: At least 2 hours before arrival.
• Vessel: At least 24 hours before arrival.
Late or missing submissions can cause refusal or delays at the border. The FDA electronically confirms acceptance once prior notice is submitted and validated.
Troubleshooting and Compliance Tips
If errors appear during submission, verifying data for accuracy and resubmitting before deadlines is critical. Use the FDA’s Web Entry system tools to review and correct drafts to avoid submission failures.
Compliance includes filing for every required shipment and keeping submission confirmations for records. Working closely with customs brokers or importers can reduce errors.
Regularly reviewing submission status and addressing FDA alerts promptly prevents shipment holds and regulatory penalties.
Related Posts
© 2026 Invastor. All Rights Reserved

User Comments