
Clinical Trials Market Size, Share, Growth, Report 2025 To 2034
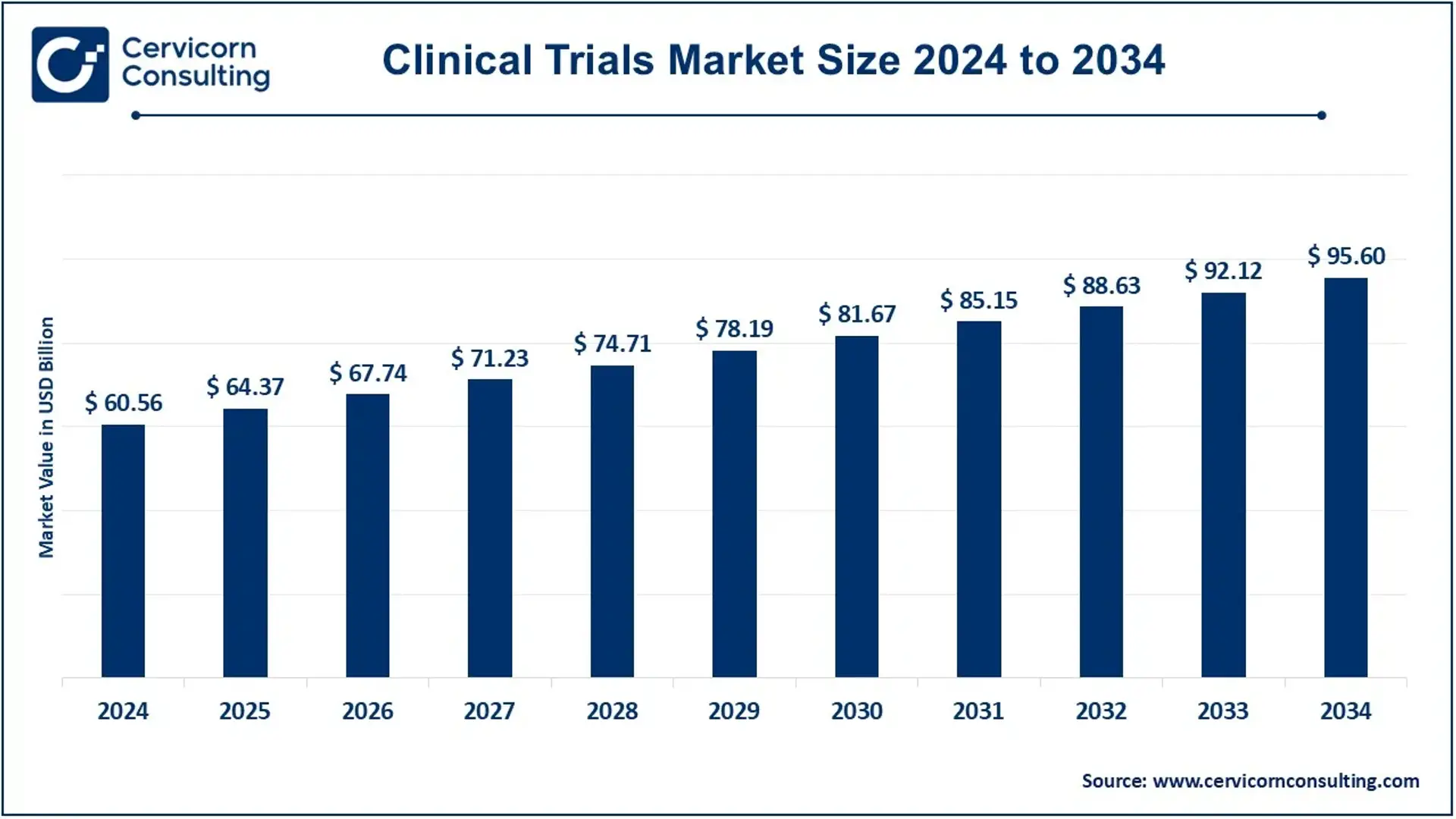
The global clinical trials market size was valued at USD 60.56 billion in 2024 and is expected to be worth around USD 95.60 billion by 2034, growing at a compound annual growth rate (CAGR) of 4.67% over the forecast period 2025 to 2034.
Clinical trials are the backbone of medical innovation. Defined as the rigorous testing of novel therapies, interventions, and drugs—including both traditional pharmaceuticals and cutting-edge biologics—clinical trials serve to establish safety and efficacy prior to market approval. A vibrant Clinical Trials Market encompasses both sponsors (pharma, biotech, academic institutions, government) and execution service providers (Contract Research Organizations, or CROs). Its size and growth mirror global healthcare investment and innovation trends.
Clinical Trials Market Report Highlights
By phase, phase III segment has accounted 48.91% market share in 2024.
By study design, interventional study has captured 76.50% market share in 2024.
By therapeutic area, oncology segment has generated 38.41% market share in 2024.
By region, North America has garnered 53.9% market share in 2024.
Get a Sample PDF of the Report:https://www.cervicornconsulting.com/sample/2308
Current Market Trends
Double-digit growth with projected ~5–9% CAGR
Market size estimated at $64 billion–$86 billion in 2023–2025, with forecast reaching $95–$150 billion by 2030–2034 at ~5–6% CAGR.
North America maintains 44–55% of the market; Asia-Pacific is fastest-growing with ~9–10% CAGR .
Rise of decentralized & hybrid trials
New models leveraging telehealth, at-home monitoring, local labs are gaining momentum, with DCT segment expected to generate $13.3 billion by 2030 .
Adoption of AI, big data, RWE
AI-enabled patient recruitment platforms, predictive modelling, and real-world data analytics are improving recruitment efficiency and optimizing trial design.
Therapeutic focus on oncology, rare diseases, chronic conditions
Oncology leads with ~38% trial share in 2024; rare-disease and orphan drug pipelines add 1.6% CAGR lift globally.
Globalization of trial sites
Trial sites increasingly based in Asia-Pacific (China, India, Japan), Latin America, and MEA to reduce costs and accelerate recruitment.
Market Drivers
Rising prevalence of chronic and rare diseases
Aging populations and NCDs spur demand for new therapeutics.
Expanding pharmaceutical pipelines and R&D investment
Higher spending, patent cliffs, and biotech proliferation drive trial volume.
Outsourcing to CROs
Sponsors seek cost efficiency and operational flexibility, fueling CRO market growth at ~8–11% CAGR.
Technological innovation
Genomics, AI, wearables, in-silico trials (computer simulations) are reshaping trial execution.
Regulatory support for decentralized design
FDA’s final DCT guidance (Sept 2024) and orphan drug incentives accelerate novel trial formats.
Market Restraints
High operational cost & regulatory complexityPhase III trials often exceed $20–50 million on complicated designs, multi-country regulatory harmonization remains burdensome .
Patient recruitment & retention challenges
80% of trials delayed due to participant issues; dropout replacements can cost $19k+ each.
Data privacy & regional infrastructure gaps
GDPR/CCPA hinder e-consent; emerging markets face coordinator shortages and training gaps.
Ethical/regulatory inconsistencies
Concerns over trial quality—especially investigator-initiated trials in China and ethical oversight in India.
Opportunities
Personalized & precision medicine
Biomarker-driven oncology and gene therapies powerful tailwinds; 60% of new drug approvals are personalized.
Emerging markets expansion
Asia-Pacific, Latin America, and MEA offer cost-efficient patient pools—China and India poised to outpace global share.
Hybrid trial models
Flexibility drives participant satisfaction; sponsor adoption removes logistical bottlenecks.
AI-driven efficiency
Enhanced trial design, lower screen failure rates, optimized operations .
Digital twin & in‑silico trials
Simulations reduce reliance on human subjects, accelerate hypothesis testing.
Regulatory incentives
Orphan drug status, rare disease grants, tax credits (e.g., Australia) support novel trial funding.
Clinical Trials Market Segmentation
By Phase
Phase I
Phase II
Phase III
Phase IV
By Indication
Autoimmune/Inflammation
Pain Management
Oncology
Cns Condition
Diabetes
Obesity
Cardiovascular
Others
By Therapeutic Area
Oncology
Infectious Diseases
Neurology
Metabolic Disorders
Immunology
Cardiology
Genetic Diseases
Women’s Health
Others
By Design
Interventional
Treatment Studies
Observational Studies
Expanded Access
Others
By End-Users
Hospitals
Laboratories
Clinics
Others
Request For Customization:https://www.cervicornconsulting.com/customization/2308
Regional Market Insights
North America
Market value: $26–27 billion (2024); ~54% global share.
Strengths: Top-tier labs, strong R&D budgets (Pfizer >$10 B, Novartis $10 B+), comprehensive regulatory support, high use of rare disease trials.
Europe
Share: ~12–27% depending on source; recent drop from 22% to 12% of global trials due to red tape .
Key hubs: Germany (top R&D spend), UK & France strong in oncology and rare-disease studies .
Barrier: Fragmented regulation despite EMA portal reforms (2022) .
Asia-Pacific
Growth: Highest CAGR ~9–10%; China now top global for trial registrations .
Drivers: Low costs, large naïve patient pools, faster trials and looser regulations .
India opportunity: Currently ~8% share—could exceed $2 billion by 2030 with regulatory improvements .
Japan/Australia: High-quality trials in oncology, regenerative medicine; Australia offers tax incentives .
Latin America & Middle East/Africa
Growth: Latin America at ~6–6.5% CAGR; MEA growing from small base .
Highlights: Brazil leads in LATAM; Saudi Arabia, UAE, and South Africa gaining regional traction .
Clinical Trials Market Top Companies
IQVIA Holdings Inc.
PPD, Inc.
ICON plc
Parexel International Corporation
Covance Inc. (LabCorp Drug Development)
Syneos Health, Inc.
Medpace Holdings, Inc.
PRA Health Sciences, Inc.
Charles River Laboratories International, Inc.
WuXi AppTec Inc.
Pharmaceutical Product Development, LLC (PPD)
Labcorp Drug Development (formerly Covance)
Worldwide Clinical Trials
In the clinical trials market, new players adopting innovation include Veristat, a CRO specializing in biotech and pharmaceutical trials with a focus on rare diseases and oncology. Additionally, Clincierge offers patient recruitment solutions through technology-driven approaches. Key players dominating the market include IQVIA, known for its comprehensive data analytics and clinical trial management solutions. PPD, with its global reach and broad therapeutic expertise, and ICON plc, renowned for its strategic consulting and operational excellence, are also significant players. These leaders maintain dominance through extensive service offerings, global presence, and strong client relationships, ensuring robust growth and market leadership.
Future Market Growth Potential
Market size forecasts range between $95–150 billion by early 2030s .
Growth accelerants include:
Hybrid/digital trial models (+1.9% CAGR impact) ;
Orphan/rare-disease pipeline growth (+1.6%)
Conclusion
The clinical trials market stands at a transformative juncture, blending advanced technology, strategic expansion, and global collaboration. While challenges like cost, recruitment, and regulatory complexity persist, the trajectory remains strongly upward. Emerging markets—especially China and India—alongside precision and patient-centric models, herald a future where trials are faster, smarter, and more inclusive. Stakeholders who adapt to digital innovation and geospatial expansion will thrive in the evolving landscape.
Purchase the Report: (Available for 3800 USD for a single-region license)-https://www.cervicornconsulting.com/buy-now/2308
About Us: Cervicorn Consulting specializes in providing expert analysis and accurate market intelligence, helping companies of all sizes make well-informed decisions.
Contact Us:
Phone: +91 7499931916
Related Posts
© 2025 Invastor. All Rights Reserved

User Comments