

Permit management in life sciences industry is indispensable. It ensures operational integrity, safety, and regulatory compliance. From pharmaceutical manufacturing to clinical research and laboratory operations, obtaining and managing permits ensures that activities are conducted within stringent legal frameworks. These frameworks are designed to protect public health, maintain environmental safety, and uphold ethical standards.
The life sciences sector is governed by a complex array of regulations, including Good Manufacturing Practices (GMP), Good Laboratory Practices (GLP), and Good Clinical Practices (GCP, etc. These regulations are enforced by various agencies, including the U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMA), and the World Health Organization (WHO).
Compliance with these regulations is a legal obligation that carries significant consequences for non-compliance, such as hefty fines, reputational damage, and even suspension of operations.
Navigating the regulatory landscape requires meticulous attention to detail and a robust permit management system. Permit management is a critical mechanism for ensuring that all activities are authorized, documented, and compliant with applicable regulations.
Qualityze Permit Management Software offers a solution to streamline this process, enabling life sciences organizations to manage permits efficiently and cost-effectively. By automating workflows, maintaining comprehensive audit trails, and ensuring compliance with regulatory requirements, Qualityze empowers organizations to meet their objectives while minimizing risks and operational disruptions.
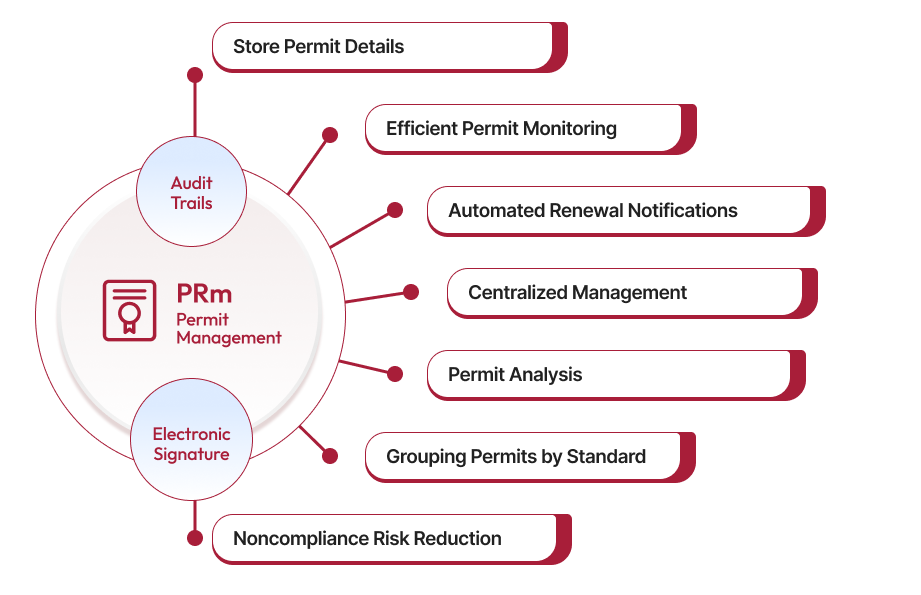
Understanding Permit Management in Life Sciences
Permit management in life sciences encompasses the processes involved in obtaining, tracking, and maintaining the necessary authorizations for various activities. These activities range from obtaining permits for laboratory experiments and clinical trials to managing permits for manufacturing processes and environmental assessments. Effective permit management ensures that all required approvals are in place before commencing any activity, thereby mitigating risks associated with non-compliance.
Key Components of Permit Management
Permit Application and Approval: The process begins with identifying the necessary permits for a specific activity, followed by preparing and submitting applications to the relevant authorities. This step often involves providing detailed information about the proposed activity, potential risks, and mitigation strategies.
Tracking and Monitoring: Once permits are obtained, monitoring their status is crucial and ensuring they remain valid throughout the activity. It includes tracking expiration dates, renewal requirements, and any conditions attached to the permits.
Documentation and Record Keeping: Maintaining accurate and organized records of all permits is essential for compliance. These records serve as evidence during audits and inspections and facilitate information retrieval when needed.
Compliance Audits and Reporting: Regular audits help assess adherence to permit conditions and identify areas for improvement. Additionally, generating reports on permit status and compliance metrics supports informed decision-making and continuous improvement efforts.
Role of Permit Management in Ensuring Compliance
Permit management plays a crucial role in ensuring compliance with these regulatory frameworks. By systematically obtaining and tracking the necessary permits, organizations can demonstrate adherence to legal requirements and avoid potential penalties. Moreover, effective permit management facilitates the identification of compliance gaps and supports corrective actions to address any deficiencies.
Challenges in Permit Management for Life Sciences
Managing permits in the life sciences industry presents several challenges:
Complex Regulatory Landscape: Navigating local, national, and international regulations can be daunting, especially for organizations operating in multiple jurisdictions.
Documentation Overload: The extensive documentation required for permit applications and renewals can be overwhelming, leading to administrative burdens and potential errors.
Tracking and Monitoring: Without a centralized system, tracking permit statuses, expiration dates, and renewal requirements across various activities and locations can be cumbersome.
Audit Preparedness: Preparing for audits necessitates the availability of accurate and up-to-date records, which can be challenging to maintain manually.
Resource Constraints: Limited personnel and resources can hinder the efficient management of permits, leading to delays and potential non-compliance.
Overcoming Challenges with Permit Management
Implementing a robust permit management system helps address these challenges by:
Centralizing Information: A centralized system consolidates all permit-related information, making it easily accessible and reducing the risk of errors.
Automating Processes: Automation streamlines workflows, reducing administrative burdens and accelerating approval timelines.
Enhancing Visibility: Real-time tracking and monitoring provide visibility into permit statuses, enabling proactive management.
Ensuring Compliance: Automated alerts and reminders help ensure that permits are renewed on time and conditions are met.
Facilitating Audits: Comprehensive records and audit trails simplify the preparation for inspections and audits.
Qualityze Permit Management Software
Qualityze Permit Management Software streamlines the permit management process in the life sciences industry. Its features include:
Centralized Repository: Stores all permit-related documents in a secure, centralized location for easy access and management.
Automated Workflows: Automates the permit application, approval, and renewal processes, reducing administrative overhead.
Real-Time Tracking: Provides real-time tracking of permit statuses, expiration dates, and renewal requirements.
Compliance Monitoring: Monitors compliance with permit conditions and regulatory requirements, alerting users to potential issues.
Audit Trails: Maintains comprehensive audit trails of all permit-related activities, facilitating inspections and audits.
Integration Capabilities: Integrates with other enterprise systems, such as Enterprise Resource Planning (ERP) and Quality Management Systems (QMS), for seamless data flow.
User-Friendly Interface: Offers an intuitive interface that simplifies user permit management process at all levels.
By leveraging Qualityze Permit Management Software, life sciences organizations can enhance their compliance posture, reduce operational risks, and achieve greater efficiency in their permit management processes.

Conclusion
Effective permit management is essential for ensuring compliance with the life sciences industry’s complex regulatory requirements. By implementing a robust permit management system, organizations can confidently navigate the regulatory landscape, mitigate risks, and maintain operational continuity. Qualityze EQMS Software of Permit Management Solution offers a comprehensive solution to streamline these processes, enabling life sciences organizations to focus on their core mission of advancing science and improving public health.
Related Posts
© 2025 Invastor. All Rights Reserved

User Comments